Quality
SafeHealth is a brand that represents premium quality. We can proudly say that our gloves have passed numerous product safety trials and tests, complying with international standardized profiles, the Quality Management System (QMS), and local regulations according to different countries. To see the certificates, please refer to the information below:
CE Marking
+moreThe CE mark indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin. In European Union, all medical device must to follow Regulation (EU) 2017/745 which is a regulation of the European Union on the clinical investigation and sale of medical devices for human use. Similarly, all personal protective equipment (PPE) should be comply to Regulation (EU) 2016/425 which require all PPE products to meet Basic Health and Safety Requirements (BHSR). Protective gloves against chemicals and micro-organisms. Determination of resistance to penetration

UKCA
+moreThe UKCA (UK Conformity Assessed) marking is a new UK product marking that is used for goods being placed on the market in Great Britain (England, Wales and Scotland). It covers most goods which previously required the CE marking, known as ‘new approach’ goods.

ISO 374-1:2016/Type B
+moreProtective gloves against chemicals (Type B, three chemicals)

ISO 374-1:2016/Type C
+moreProtective gloves against chemicals (Type C, one chemical)

ISO 374-5
+moreProtective gloves against chemicals and micro-organisms. Determination of resistance to penetration.
EC 1935/2004
+moreThe international symbol for 'food safe' material. The symbol identifies that the material used in the product is safe for food contact. The regulation, Regulation (EC) 1935/2004, Regulation (EC) 2023/2006, and Regulation (EC) 10/2011 are applicable to all products of ours which are intended for food contact. Special instructions should be observed for safe and appropriate use.

ISO 9001
+moreISO 9001 specifies requirements for a quality management system when an organization: (a) needs to demonstrate its ability to consistently provide products and services that meet customer and applicable statutory and regulatory requirements, and (b) aims to enhance customer satisfaction through the effective application of the system, including processes for improvement of the system and the assurance of conformity to customer and applicable statutory and regulatory requirements. Third-party certification bodies provide independent confirmation that organizations meet the requirements of ISO 9001.

ISO 13485
+moreISO 13485:2003 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet the requirement of safety, performance, quality, regulation applicable to medical devices and related services.

ISO 14001
+moreISO 14001 is an international standard which specifies requirements for an environmental management system. It helps an organization develop and implement a policy and objectives which help organization maintain commercial success without neglecting their environmental responsibilities.

BRC
+moreBRC-Personal Care and Household covers formulated and fabricated products which typically have higher hygiene requirements due to the nature and usage of products. Examples include cosmetics, medical devices, nappies, food wrap and household cleaners.

SA8000
+moreSA8000 measures social performance in eight areas important to social accountability in workplaces, anchored by a management system element that drives continuous improvement in all areas of the Standard.

ISO 9001:2015
+moreThe new ISO 9001:2015 management system standard helps ensure that consumers get reliable, desired quality goods and services.
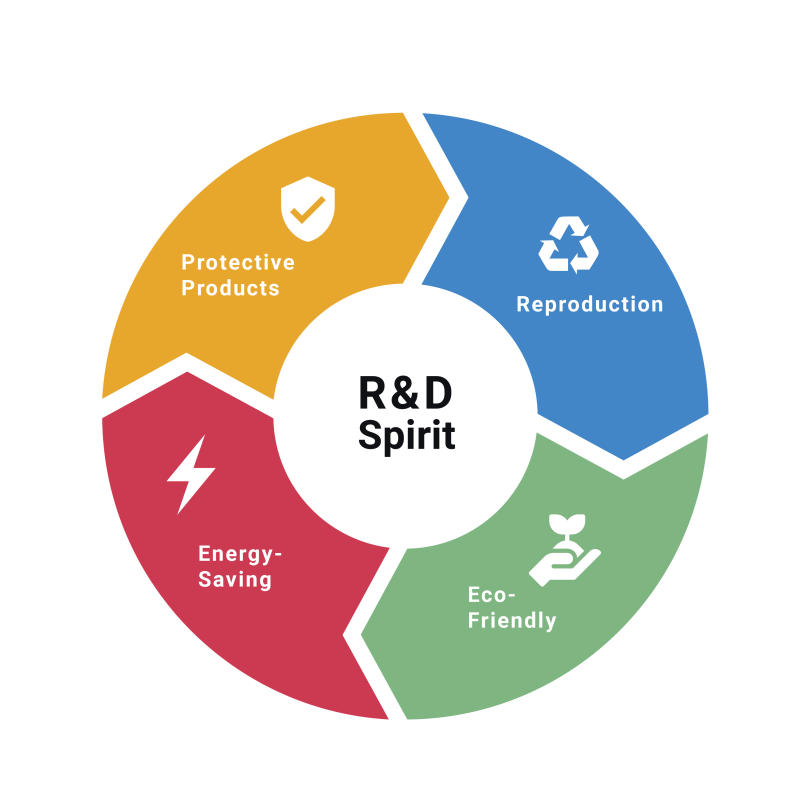
Technology
The R&D spirit of SafeHealth is to provide our customers with the high-quality medical and industrial gloves they deserve. We also focus on and carefully monitor the production of our protective products to ensure they are eco-friendly and energy-saving. The company’s goal is to lead the consumption trend whilst exploring and meeting the unfulfilled needs of the customer.

Think outside the box
SafeHealth’s expertise, innovative technology, and communication with our customers has helped the R&D team create a number of world first inventions, as well as diversified Personal Protective Equipment (PPE). As well as being ergonomically designed, SafeHealth products are not only eco-friendly by using biobased materials, but also extremely hygienic due to the glove’s antimicrobial qualities, which we understand is of great importance especially after the epidemic.
SafeHealth is dedicated to set the highest standards of quality and technology in the world. We are continually striving to be at the forefront of product development in everything we do.

Sustainable Business Philosophy
SafeHealth strives to maintain the growth of the natural environment and the minimization of artificial contaminants on Mother Earth. Driven by that mission, we design products that can not only reduce production costs, but also effectively reduce environmental pollution caused by the usage of traditional plastic. Designing products that are consistent with environmental protection and sustainability is a value our company lives by. In doing so, SafeHealth joins the effort to create a greener world.

